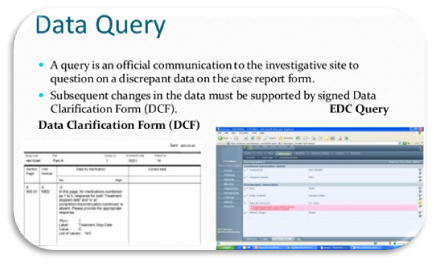
A Data Clarification Form (DCF) or Data Query Form (DQF) is a questionnaire specifically used in clinical research.
The DCF is the primary data clarification tool from the trial sponsor or Contract Research Organization (CRO) towards the investigator to clarify discrepancies and ask the investigator for clarification. The DCF is part of the data validation process in a clinical trial.
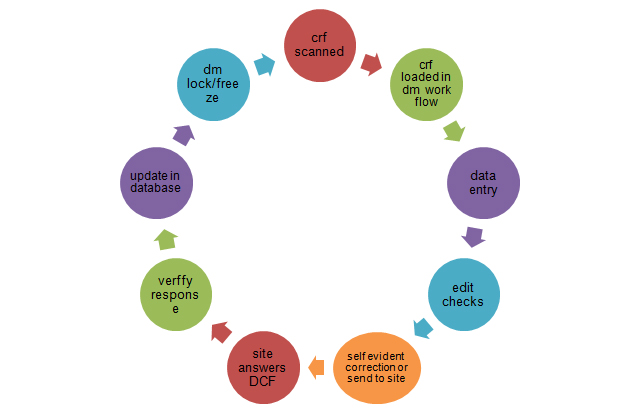
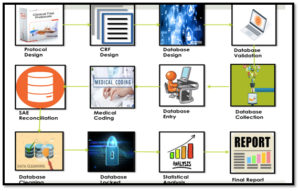
In clinical trials, the data validation step and asking the investigator for clarification of discrepancies (data cleaning) is an important step towards creating a clean dataset suitable for statistical analysis of the clinical trial outcome.
A Data Clarification Form (DCF) or Data Query Form (DQF) is a questionnaire specifically used in clinical research. The DCF is the primary data clarification tool from the trial sponsor or Contract Research Organization (CRO) towards the investigator to clarify discrepancies and ask the investigator for clarification. The DCF is part of the data validation process in a clinical trial.

DATA CLARIFICATION FORM (DCF) Example
| Protocol No: | Country: | Site: Kinshasa |
| PI name: | Originator: | Date written: |
| DCF No: | Patient Number: | Patient initials: |
| CRF page | Question | Response |
- Respond to the question in the response box to the right
- Sign and date this form
- Attach a copy to the patients’ CRF on site
- Return the original DCF back to Data Management within 72h
Adding Multivariate Discrepancy’s Procedure Variables to a DCF
Multivariate discrepancies are created by Validation Procedures that compare multiple question responses as variables.
Adding a Discrepancy to a DCF
When you add discrepancies after you have created the DCF, you can only add discrepancies that match the above criteria and are not already ACTIVE on the current DCF.
Removing a Discrepancy from a DCF
You can remove a discrepancy from a DCF by selecting the discrepancy and clicking the Remove button..
If you remove a discrepancy from a DCF that has not yet been SENT, the discrepancy is deleted from the DCF. If the DCF has progressed beyond the SENT status, the discrepancy is still visible in the report, but its status is RELEASED.
DISCREPANCY MANAGEMENT