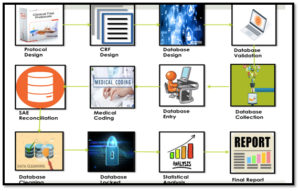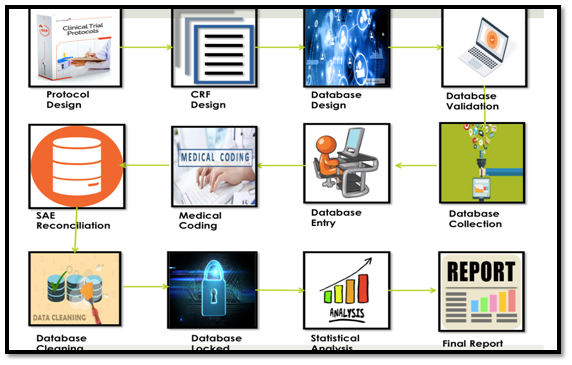
Steps in CDM
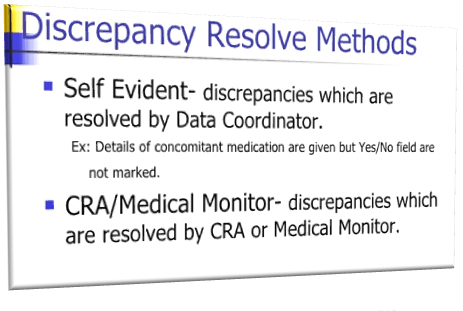
Self-evident corrections are changes to data or query resolution, that can be easily corrected on the basis of information entered on the Case Record Form (CRF), without sending a query to the site. Some of the most common self-evident corrections are spelling errors.
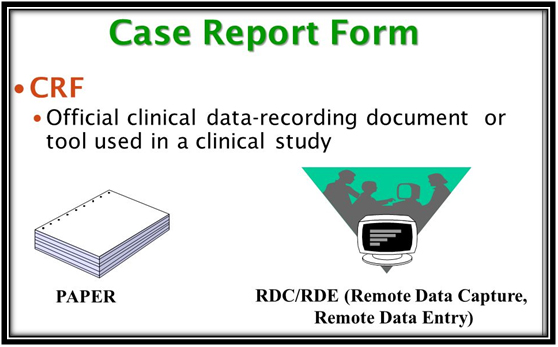
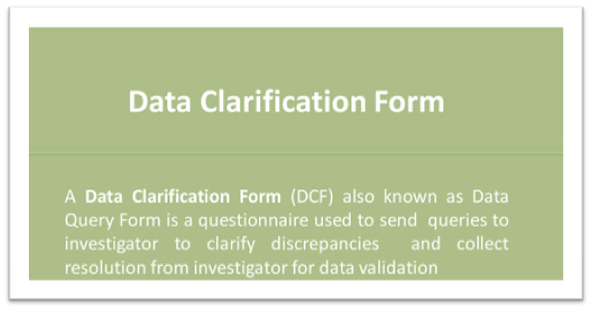
A section should specify the criteria for self-evident corrections, and also identify data management team members that can make corrections to the data (as necessary). These corrections must be clearly documented and audited. The DMP should also include a list of approved self-evident corrections.
A list of corrections to the case report form that can be made by the sponsor’s data management staff without the requirement for case-by-case referral to the investigator
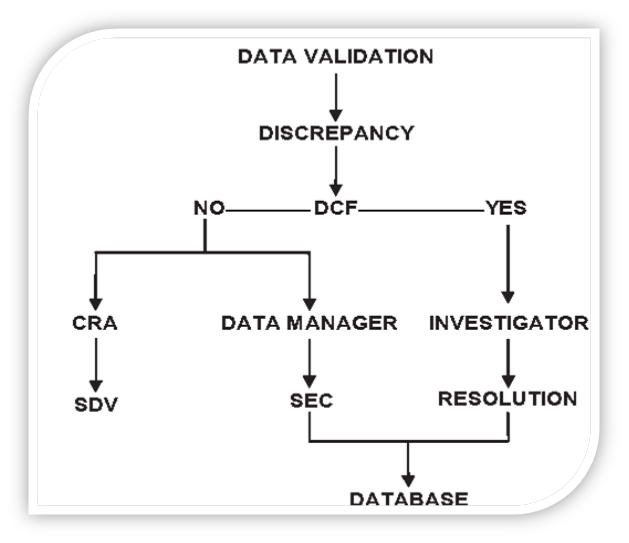
For example, if a case report form page lists concomitant medications taken by a patient but the box stating ‘Are there any medications this cycle?’ is blank, the box may be ticked by the data manager.
A list of such data correction conventions should be agreed by the investigator prior to data management activities taking place.