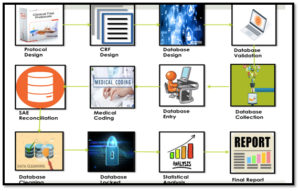
Firstly let us understand what is clinical trial
Clinical trial in simple terms validates the safety and efficacy of the drug to enter the market for sale.Clinical trial undergoes various phases increasing the number of participant to the next step.
Epidemic-The occurrence of more cases of a disease that would be expected in a communt or a region during a given time period.
Example

- In 2003 A sudden outbreak of a disease such as SARS
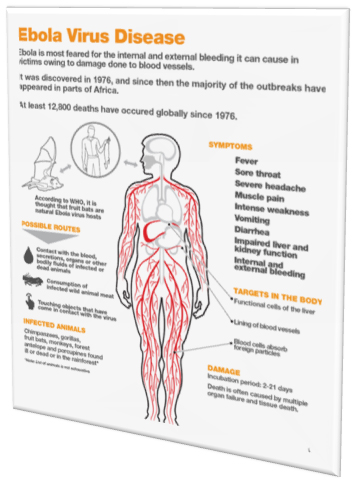
- In 2014 Ebola virus ,Africa
- In 2019 Novel Corona Virus, China
Randomized Clinical Trials are the fastest way to identify vaccines in an outbreak,but more collaboration is needed among international researchers including Contract Research organizations (CROs).
In October 2015, the office of assistant secretary for preparedness and response, the national institute of allergy and infectious disease and the USFDA asked national academics of sciences, engineering and medicines to review and analyze clinical trial conducted in West Africa during 2014 Ebola epidemic.
The committee determined randomized clinical trials are both ethical and preferable in the context of an epidemic as RCT provide fastest way to identify beneficial treatment and vaccines while minimizing risk.
RCT in epidemics are challenging but safety and effective drug can be beneficial in saving a lot of lives.
Indian Council of Medical Research have released National Ethical Guidelines For Biomedical And Health Research Involving Human Participants there is an section on “Research During Humanitarian Emergencies And Disasters
A humanitarian emergency or disaster is an event or series of events that represents a critical threat to the health, safety, security or well being of a community or other large group of people, usually covering a wide land area.
For the purpose of these guidelines, humanitarian emergencies and disasters include both man-made and natural ones, some of which occur at periodic frequency.
Emergencies, such as an earthquake, flood, mass migration, conflict and outbreak of disease, leading to substantial material damage affecting persons, communities, society and state(s), create an imbalance between capacity and resources to meet the needs of the survivors or the people whose lives are threatened during that period.


Research is necessary in such circumstances to enable provision of efficient and appropriate health and humanitarian response during the ongoing emergency and to be able to plan for future emergency situations.
Local, national or international responses and preparedness, without interfering with measures to control the crisis or ecology, is the key to reducing morbidity and mortality in such events.
Humanitarian emergencies raise complex issues. The health system, communications, research infrastructure, and research governance frameworks may be adversely affected during such situations, which create challenges for the feasibility and oversight of conduct of research.
While there may be a need to undertake research quickly, this should not impact scientific validity and the need to uphold ethical requirements.
Close attention should be paid to the effect of the emergency on perceptions of ethical questions, altered or increased vulnerabilities, provider–patient and researcher–participant relationships, issues related to integrity of studies and ethical review processes.
A unique challenge would be the response to rapidly evolving health needs or priorities of those impacted by the humanitarian emergency when the research cannot be conducted outside the humanitarian emergency situation.
Designing or adopting innovative relevant research, based on rapidly evolving scientific and ethical uncertainties, which is expected to yield scientifically valid results is another significant challenge.
The other challenges are inadequate time to design a study and lack of infrastructure facilities and resources to conduct it within a disrupted physical-socio-cultural environment.
The role of ECs in such circumstances is very important in reviewing protocols prepared for such emergency situation(s). Responsiveness to the situation, supervision, training and prevention of heightened risk of violence are other factors to be considered and planned.
A natural disaster of cyclical frequency is an expected phenomenon. The following will be acceptable if a research is planned to study various implications on humans and ecological effects on humans in these circumstances.
Researchers and sponsors could make arrangements about research questions to be addressed in the design, collection of samples and data, and sharing mechanisms much in advance of a future humanitarian emergency.
Researchers could screen available and/or relevant draft research protocols to expedite the review process. The EC could review proposals prior to the occurrence of the emergency and determine who could be an acceptable LAR in the absence of intended LARs (authorized/ acceptable) in such situations.